Iron as sustainable energy carrier
Background:
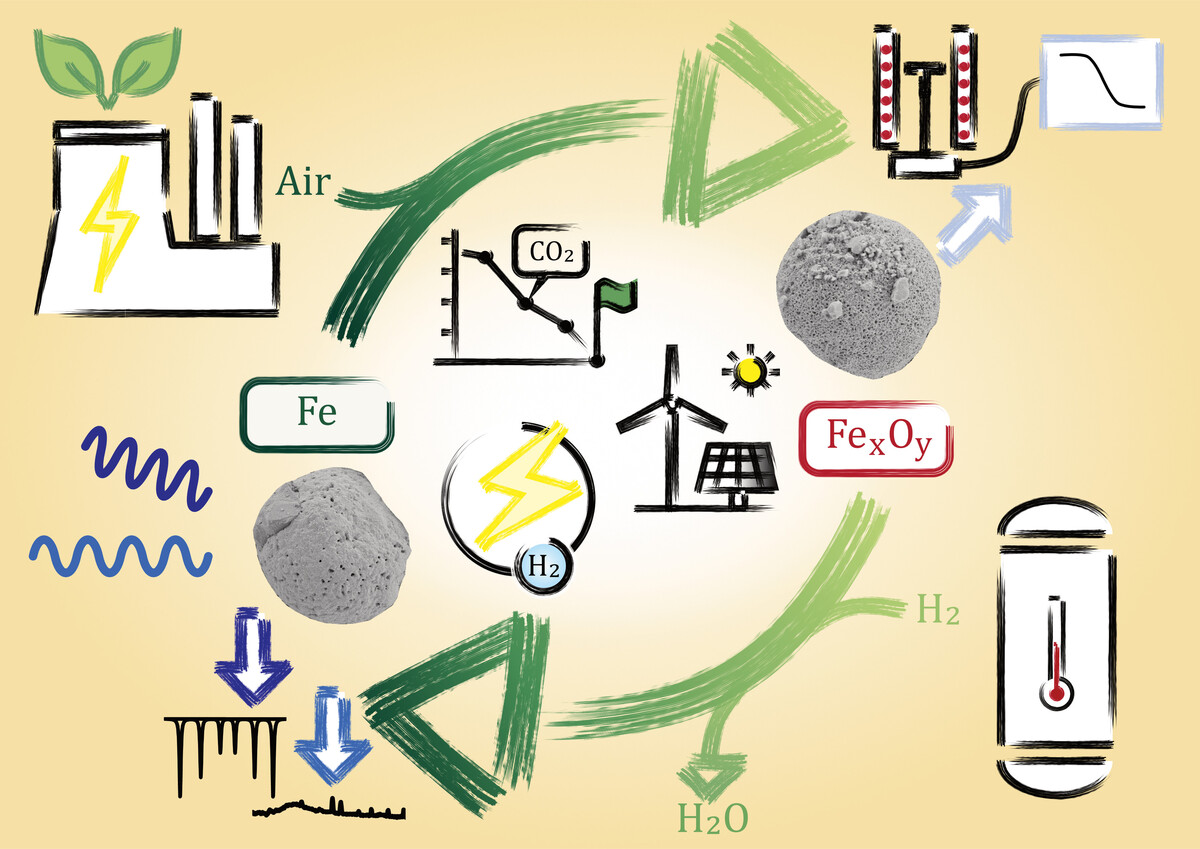
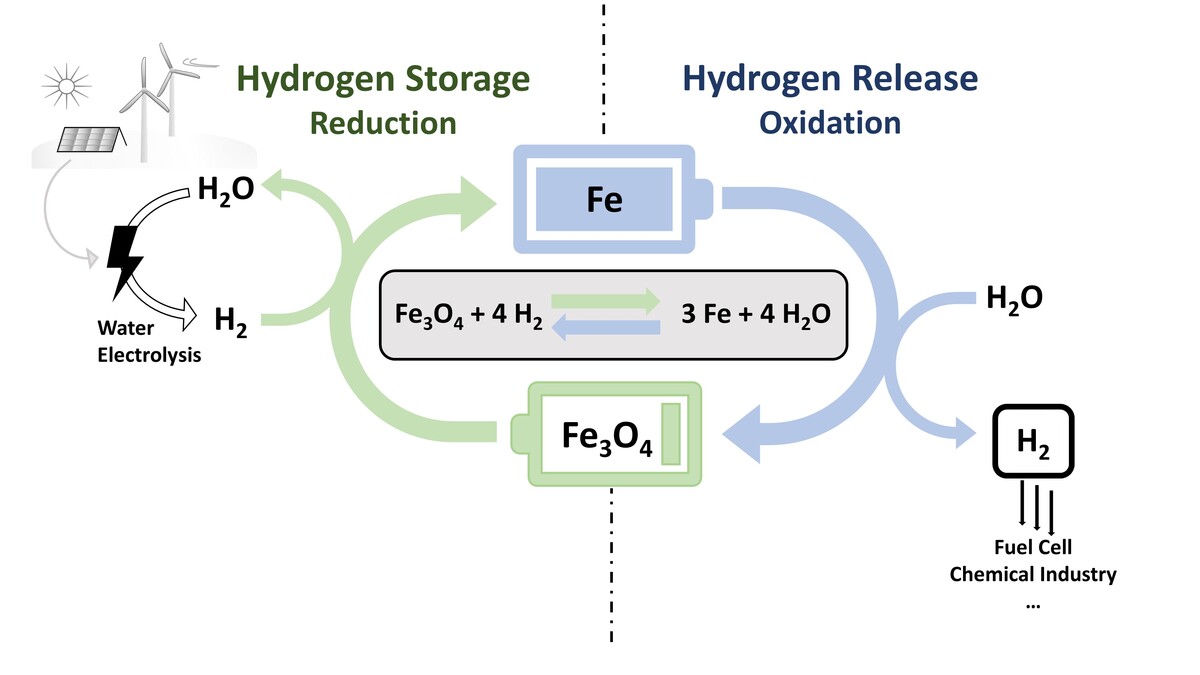
Reactive metals such as aluminum, sodium, magnesium, zinc, and iron are gaining increasing attention as carbon-free chemical energy carriers in a circular energy economy. Among these, iron is particularly promising due to its high volumetric energy density, non-toxicity, abundance, ease of transport, and suitability for long-term storage. When reduced with hydrogen, iron can store renewable energy and release it again through oxidation, offering both temporal and spatial flexibility.
Two main process routes are currently being investigated for iron-based energy storage systems: the dry cycle, in which reduced iron is oxidized with air to release thermal energy, and the wet cycle, in which iron is oxidized with steam to release hydrogen. Both approaches rely on a cyclic redox process and pose distinct scientific and technological challenges that must be addressed to enable efficient and scalable applications. The project Clean Circles investigated the iron cycle with an integrated approach in cooperation with the TU Darmstadt and further partners.
Projects:
Reduction Behavior of Oxidized Iron Particles
In a collaborative project with the Institute of Mechanical Process Engineering and Mechanics and the Engler-Bunte-Institute we focus on the reduction of combusted iron particles using hydrogen as a key step in the energy storage cycle. This project aims to investigate the reduction mechanisms and kinetics of iron oxide reduction, which is critical for optimizing the performance of iron as a chemical energy carrier. We combine experimental work with advanced modeling and simulation to investigate the detailed reaction pathways, kinetics, and thermodynamic conditions governing the reduction process. By applying computational models, we aim to predict the behavior of iron oxide particles under various reaction conditions, and optimize the parameters for maximum efficiency. The modeling efforts help in designing scalable processes, identifying key factors that influence the reduction rate, and predicting the material performance over time.
This integrated approach, combining experiments and simulations, will provide a comprehensive understanding of the reduction process and guide future developments to improve the efficiency and scalability of iron-based energy storage systems.
Hydrogen Storage via Steam-Iron Process
In a parallel project, we study the steam-iron process (wet cycle) as a method for indirect hydrogen storage. This involves the cyclic reduction of magnetite (Fe3O4) to metallic iron using hydrogen, followed by its oxidation with steam to release hydrogen. A key challenge in this process is the degradation of material performance over repeated cycles, primarily due to sintering effects. The project investigates how the choice of dopants and synthesis methods can improve the stability and efficiency of iron-based materials over time. Understanding the interplay between material composition, microstructure, and cyclic behavior is essential for developing robust systems for large-scale hydrogen storage.
Contact: Anna Knapp, Carola Kuhn, Olaf Deutschmann
Funding: KIT Strategiefonds, DFG
Collaborations: Clean Circles, MVM, EBI-VBT
Publications:
| Micron-sized iron particles as energy carrier: Cycling experiments in a fixed-bed reactor C. Kuhn, M. Kirn, S. Tischer, O. Deutschmann, Proc. Combust. Inst. 2024, 40, 105207. https://doi.org/10.1016/j.proci.2024.105207 |
| Iron as Recyclable Metal Fuel: Unraveling Oxidation Behavior and Cyclization Effects Through Thermogravimetric Analysis, Wide- Angle X-ray Scattering and Mössbauer Spectroscopy C. Kuhn, A. Knapp, M. P. Deutschmann, J. Spielmann, S. Tischer, U. I. Kramm, H. Nirschl, O. Deutschmann, ChemSusChem 2024, 17, e202400351. https://doi.org/10.1002/cssc.202481504 |
| Iron as recyclable energy carrier: Feasibility study and kinetic analysis of iron oxide reduction C. Kuhn, A. Düll, P. Rohlfs, S. Tischer, M. Börnhorst, O. Deutschmann, Appl. Energy Combust. Sci. 2022, 12, 100096. https://doi.org/10.1016/j.jaecs.2022.100096 |