Multiphase chemical reactions in SCR systems
Background:
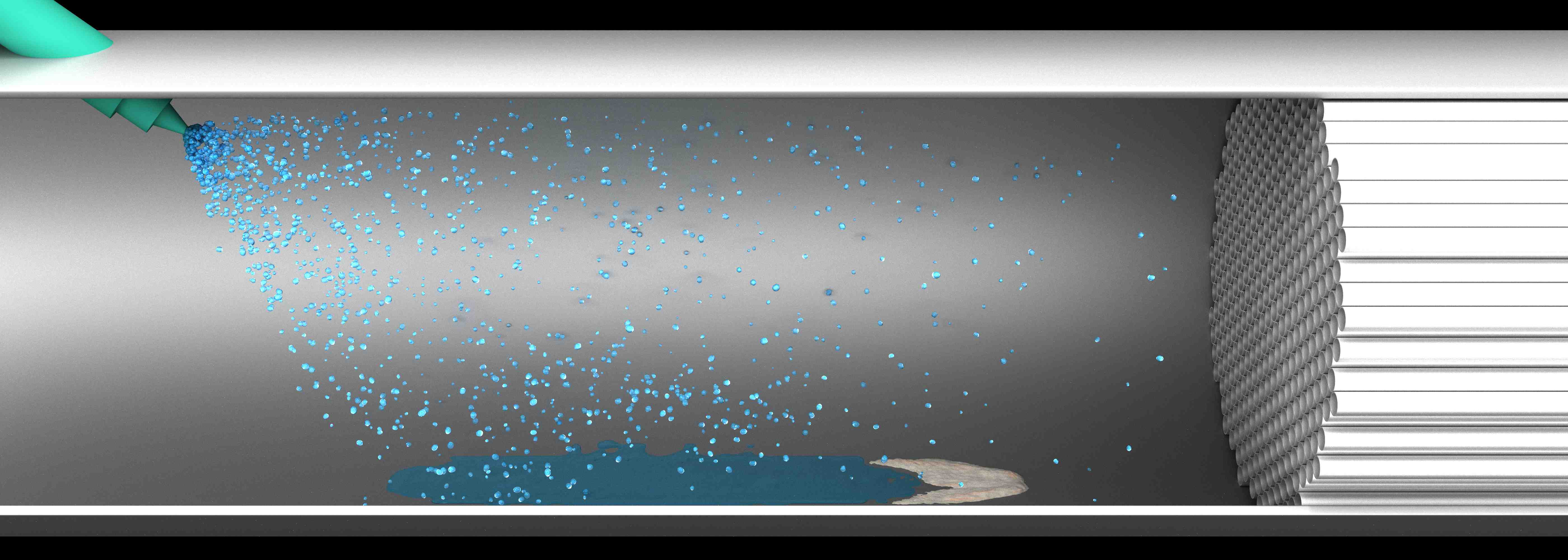
The optimization of technical systems in emission control is a challenging task. Due to interactions of multiphase chemical reactive flows with solid walls, problems arise for the whole system. This holds true particularly for selective catalytic reduction (SCR), one of the most promising solutions on exhaust aftertreatment to decrease nitric oxide (NOx) emissions of vehicles and engines utilizing diesel. In those aftertreatment systems, a ureawater-solution is used as an ammonia (NH3) precursor for removing NOx. Urea is injected in front of the SCR catalyst. Due to transient operating conditions and space limitations, evaporation can be incomplete resulting a liquid wall film and possibly in undesired urea byproducts. Urea derived deposits can increase backpressure, reduce ammonia uniformity or block the catalyst channels. Therefore, precise understanding of multi-phase reactions in the tailpipe and experimental data for predictive modeling is required.
Project:
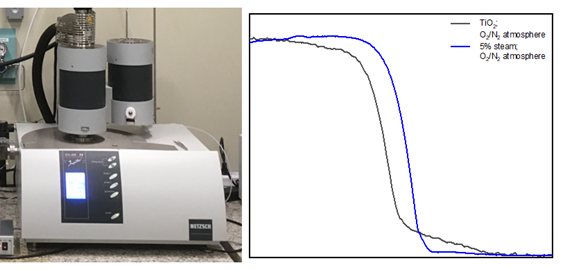
This project focuses on catalytic and non-catalytic isocyanic acid (HNCO) reactions in the tailpipe, as it is the most important intermediate in terms of harmful deposit formation. Therefore, the concentration profiles and deposits are studied within a hot gas generator setup, which was especially designed for this project. The setup is optically accessible through a quartz glass window in the injection area. In this area it is possible to observe liquid film formation and deposits via video camera or possible laser absorption spectroscopy. Downstream, a SCR catalyst is installed. By adapting the SpaciPro technology, the channels of the catalyst can be accessed by capillaries. This extension on the hot gas setup ensures the possibility to measure axially resolved concentration and temperature profiles in the channels. Concentration of the gas phase species during the SCR process and the HNCO decomposition are determined by means of FTIR spectroscopy or mass spectrometry. Furthermore, the resulting deposits and the chemical process of urea decomposition are analyzed by means of ex situ analysis for better understanding. Applied methods are thermogravimetrical analysis (TGA), high-pressure liquid chromatography (HPLC) and nuclear magnetic resonance spectroscopy (NMR). These studies have lead to substantial improvement in understanding and modeling the urea decomposition mechanism during the past years.

Contact: , , Prof. Dr. Olaf Deutschmann
Funding:

