Partial Oxidation of Higher Hydrocarbons
Marco Hartmann, Thorsten Kaltschmitt, Olaf Deutschmann
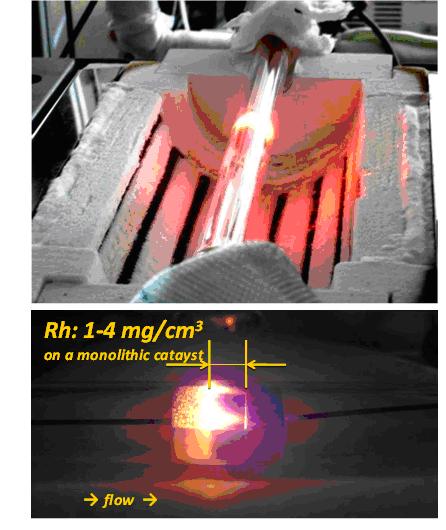
Figure 1: CPOX of n-octane
(4% fuel, 16% O2, 80% dilution N2, autothermal operation, contact time ~ 5 ms)
Motivation:
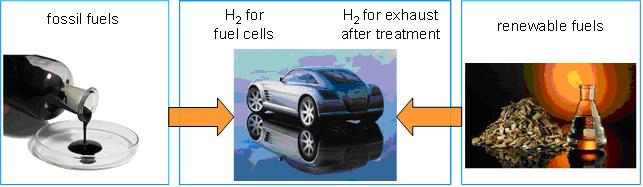
The reforming of logistic transportation fuels such as gasoline and diesel by Catalytic Partial Oxidation (CPOX) to hydrogen or synthesis gas has gained significant attention since it allows the self-sufficient operation of fuel cells for mobile and on-site power generation.
Besides high selectivity to hydrogen (SH2 > 90%), the reaction distinguishes by short contact times (milliseconds) and low ignition temperatures on rhodium coated catalysts (Ti » 250°C). Whereas the development of a hydrogen infrastructure for fuel cells is considered as cost intensive and complex, CPOX technology has the potential to feed fuel cells via existing routes of supply. By utilizing the superior electrical efficiency of fuel cells in comparison to conventional generators, CPOX is enabling their evolution from a niche product to a mass market for a more sustainable and innovative production of electric power.
Moreover, hydrogen and synthesis gas, produced on-board can also be used as additives to the internal combustion engine during the cold start-up period, which drastically reduce the formation of nitrogen oxides (NOx) and for exhaust-gas after-treatment through selective catalytic reduction (SCR) of NOx.
For a reliable application and optimization of CPOX a better understanding of the complex interactions between gas phase and surface reactions in compact CPOX reforming reactors is necessary. This effort demands a combined approach of both experiments and detailed modeling to achieve an advanced resolution and allocation of chemical and physical processes in the catalytic reactor. Both issues are addressed in the research performed in our group.
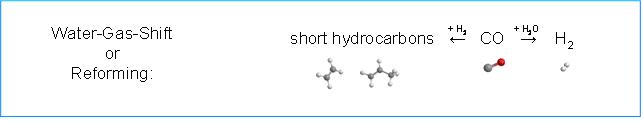
Figure 2: Upper panel - Conversion of small amounts of hydrocarbons leads to high yields of hydrogen (1 mol C10H22 performs up to 21 mol H2. Lower panel - potential of CPOx of higher hydrocarbons - generating hydrogen e.g. for on-board applications from fossil and renewable fuels
Research affords:
Experimental
A newly developed experimental set-up allows accurate mixing of fuels with boiling points up to 260°C with synthetic air to feed the catalyst with a homogeneous, pulse free reactant flow and employ a uniform temperature profile. The gaseous fuel and synthetic air flow are metered with high accuracy and reproducibility and rapidly mixed below auto ignition temperatures to avoid gas phase reactions upstream the catalyst. All relevant species present in the product stream are detected by a variety of simultaneously applied analysis tools: FT-IR, MS, GC/MS, Magnos. A process FT-IR spectrometer with high-optical-throughput sampling cell was expended by a sector field mass spectrometer and a paramagnetic analyzer for the infrared inactive species hydrogen and oxygen. By this combined method, the instantaneous exploration of the reactive flow with closed carbon, hydrogen, and oxygen balances is possible and allows time resolved monitoring of more than 12 species. Further investigation of carbon species are performed by GC/MS.
Modeling:
The complex mixture of a large number of intermediates and radicals (over 200 chemical species) formed in the complex heterogeneous and homogeneous reaction network presents a challenge for the numerical simulation of the reactor. Therefore, we recently developed a computer code for the two-dimensional simulation of reactive channel flows coupled with elementary-step reaction mechanisms. The resulting DAEs system is solved by an implicit method, based on the backward differentiation formulas (BDF), with variable order, variable step-size control methods and an efficient modified Newton method for the solution of the nonlinear equation arising from the BDF discretization. For the first time, a code is available that can solve over two hundred species equations and thousands of reactions in two phases for a two-dimensional flow problem (single monolith channel.
Publications:
[1] Hartmann, M. Erzeugung von Wasserstoff mittels katalytischer Partialoxidation höherer Kohlenwasserstoffe. Dissertation, University of Karlsruhe (2009).
[2] Hartmann, M., Deutschmann, O., Lichtenberg, S., Hebben, N. & Zhang, D. Experimentelle Untersuchung der katalytischen Partialoxidation von Modellkraftstoffen unter definierten Randbedingungen.
Chemie Ingenieur Technik 81, 909-919 (2009).[3] Hartmann, M., Maier, L., Minh, H. D. & Deutschmann, O. Catalytic Partial Oxidation of iso-Octane over Rhodium Catalysts: An Experimental and detailed Numerical Study. Combustion and Flame submitted (2009).
[4] Hartmann, M., Maier, L., Minh, H. D. & Deutschmann, O. Catalytic Partial Oxidation of higher hydrocarbon fuels over rhodium catalysts: An Experimental and
Numerical Study. 20th International Symposium on Chemical Reaction Engineering, oral contribution (Kyoto, Japan, 2008).[5] Hartmann, M., Kaltschmitt, T. & Deutschmann, O. Catalytic partial oxidation of higher hydrocarbon fuel components on Rh/Al2O3 coated honeycomb monoliths. Catalysis Today In Press.
[6] Hartmann, M., Kaltschmitt, T. & Deutschmann, O. Catalytic partial oxidation of higher hydrocarbon fuel components on Rh/Al2O3 coated honeycomb monoliths. 3rd International Symposium on Structured Catalysts, oral contribution (Iscre, Italy, 2009).
[7] Baldea, G., Maier, L., Hartmann, M., Riedel, U. & Deutschmann, O. Kinetic modeling of the partial oxidation of large aliphatic hydrocarbons using automatically generated mechanisms. 32nd International Symposium on Combustion, WIP-Poster W5P066, (Montreal, Canada, 2008).
[8] Baldea, G., Maier, L., Hartmann, M., Riedel, U. & Deutschmann, O. Automated Mechanism Generation and Kinetic Modeling for Partial Oxidation of Iso-Octane. Proceedings of the 4th European Combustion Meeting, Paper 172 (Vienna, Austria, 2009).
[9] Baldea, G., Maier, L., Hartmann, M., Riedel, U. & Deutschmann, O. Automated Mechanism Generation and Kinetic Modeling for Partial Oxidation of Iso-Octane. Proceedings of the 4th European Combustion Meeting, Paper 172 (Vienna, Austria, 2009).
[10] Bhattacharjee, T., Maier, L., Riedel, U., & Deutschmann, O. First Principles Study of Ethane Dehydrogenation on a Model Catalyst Surface. AIChE Annual Meeting, poster presentation (Nashville, USA, 2009)
[11] Bhattacharjee, T., Inderwildi, O. R., Jenkins, S. J., Riedel, U. & Warnatz, J. Oxidation of hydrocarbons at surface defects: Unprecedented confirmation of the oxomethylidyne pathway on a stepped Rh surface. Journal of Physical Chemistry C 112, 8751-8753 (2008).
[12] Thormann, J., Maier, L., Pfeifer, P., Kunz, U., Deutschmann, O., Schubert, K. Steam reforming of hexadecane over a Rh/CeO2 catalyst in microchannels: Experimental and numerical investigation. International Journal of Hydrogen Energy 34, 5108-5120 (2009).
[13] Deutschmann, O., Tischer, S., Kleditzsch, S., Janardhanan, V.M. , Correa, C., Chatterjee, D., Mladenov, N., Mihn, H. D. DETCHEM Software package, 2.1 ed., www.detchem.com, Karlsruhe (2007).
[13] Minh, H. D., Bock, H. G., Tischer, S. & Deutschmann, O. Fast solution for large-scale 2-D convection-diffusion, reacting flows. Computational Science and Its Applications - Iccsa 2008, Pt 1, Proceedings 5072, 1121-1130 (2008).
[14] Minh, H. D., Bock, H. G., Phu, H. X. & Schloder, J. P. Fast Numerical Methods for Simulation of Chemically Reacting Flows in Catalytic Monoliths. Modeling, Simulation and Optimization ofComplex Processes, 371-380 (2008).
[15] Minh, H. D., Bock, H. G., Tischer, S. & Deutschmann, O. Optimization of two-dimensional flows with homogeneous and heterogeneously catalyzed gas-phase reactions. Aiche Journal 54, 2432-2440 (2008).
[16] Hartmann, M., Maier, L., Minh, H. D. & Deutschmann, O. Experimental and Numerical Studies of Catalytic Partial Oxidation of Higher Hydrocarbons over Rhodium. 14th International Conference on Catalysis, oral contribution, Seoul, South Korea (2008).