laminar premixed flames
Example: Calculation of a confined stoichiometric premixed H2/O2-flame
In a premixed flame the fuel and the oxidant are molecularly mixed before the combustion process takes place.
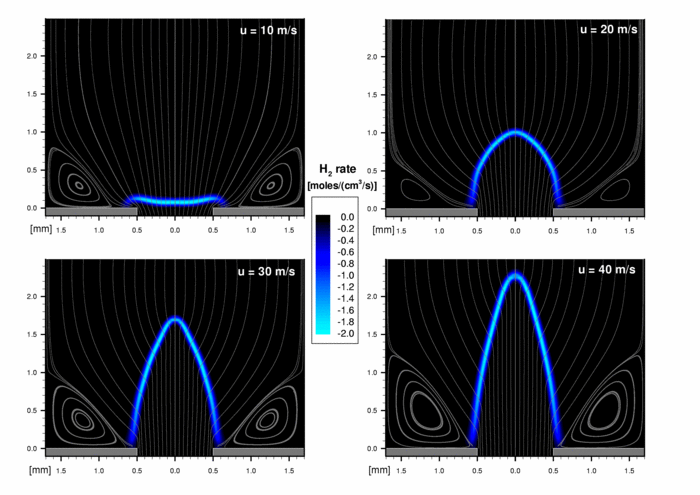
The idealized shape of the reaction zone of a laminar premixed flame is a cone. The height of the cone represents the flame length, and depends on the velocity at the burner outlet. For real flames the geometry shows deviations from the ideal shape (tip and base anomaly).
The calculations were done with the CFD-Code TASCFLOW where a 2-step mechanism for the fuel conversion was implemented. The combustion chamber had an axial length of 4 mm and a radius of 1.7 mm. The nozzle diameter was 0.5 mm. All wall temperatures had been fixed to 300 K. The velocity at the inlet varied between 10 and 40 m/s.
The first figure shows the H2 consumption rate and some streamlines. The streamlines indicate the recirculation zones at the corners of the chamber. In the cold region of the premixed fluid the streamlines are all parallel. In the preheating zone they turn versus the reaction zone and afterwards they diverge due to the expansion. The greater the inlet velocity, the longer the flame cone. At an outlet velocity of 10 m/s the flame is flat. The flame speed of the mixture is ca. 9 m/s. Therefore the flame in this case is situated just before flashback. The quenching effect due to the cold walls is reproduced well. The rounding of the flame tip is also included.
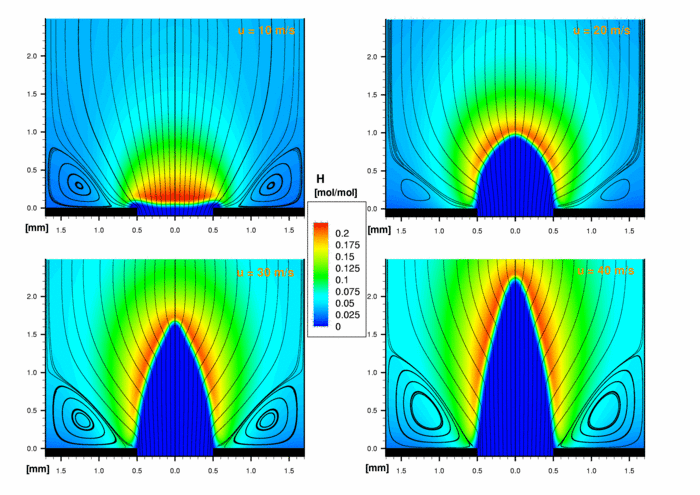
The second figure shows the concentration of the H radical which has a maximum value of 20 vol%. The indicate show the typical behaviour of this intermediate species. H is produced in the main reaction zone and is consumed in the outburn zone.
Figures:
1. H2 consumption rate of a confined stoichiometric premixed H2/O2-flame.
2. H concentration of a confined stoichiometric premixed H2/O2-flame.