Bio-based and fine chemicals

In our Bio-based and Fine Chemicals research, we develop catalytic routes that transform renewable carbon into high-value acids, monomers, and functional intermediates used across everyday products and industrial manufacturing. By designing selective solid catalysts and oxidation-centered processes, we enable efficient conversion of biomass-derived molecules into versatile building blocks—linking sustainable feedstocks with the performance and scale required for modern chemical production.
Dr. Erisa Saraci
IKFT/ITCP
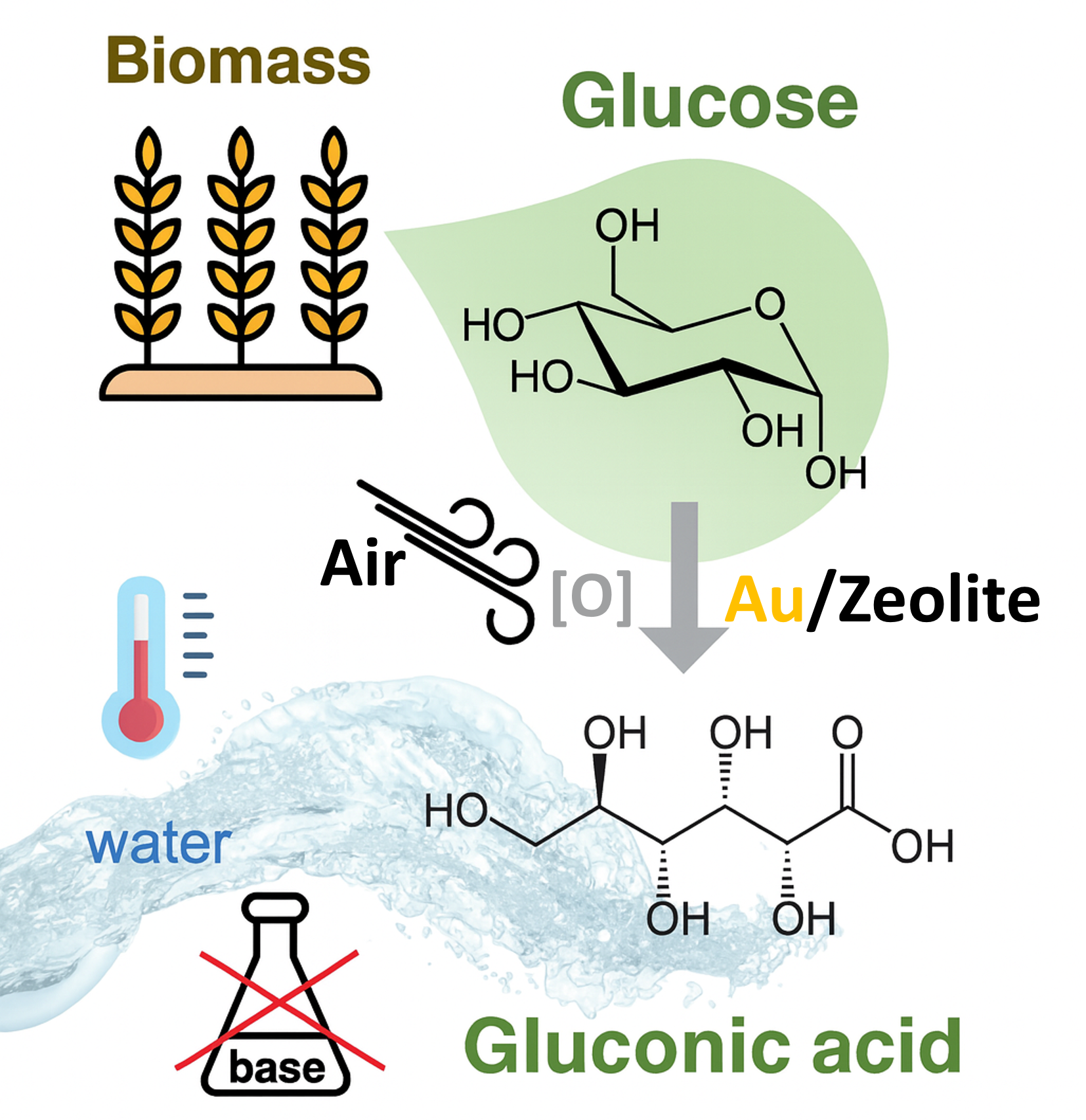
Base-Free Glucose Oxidation on Zeolite-Supported Gold Catalysts
Replacing fossil-derived resources with renewable feedstocks requires efficient catalytic routes that convert abundant sugars into green acids used in food, pharma, and polymer value chains. A key challenge in glucose oxidation is achieving high selectivity without added base, while maintaining long-term catalyst stability under aqueous conditions. Deactivation by metal leaching, nanoparticle sintering, and uncontrolled surface chemistry can rapidly erode performance. Our approach combines tailored Au nanoparticle synthesis with zeolitic supports that provide anchoring, dispersion control, and tunable acidity to promote the desired reaction pathway while suppressing side reactions. In this way, we advance robust, base-free oxidation concepts that enable sustainable production of sugar-derived acids.
Scientist: F. Zormpa
Related Publications
Advancing biomass valorization with zeolite catalysts: Focus on oxidative transformations.
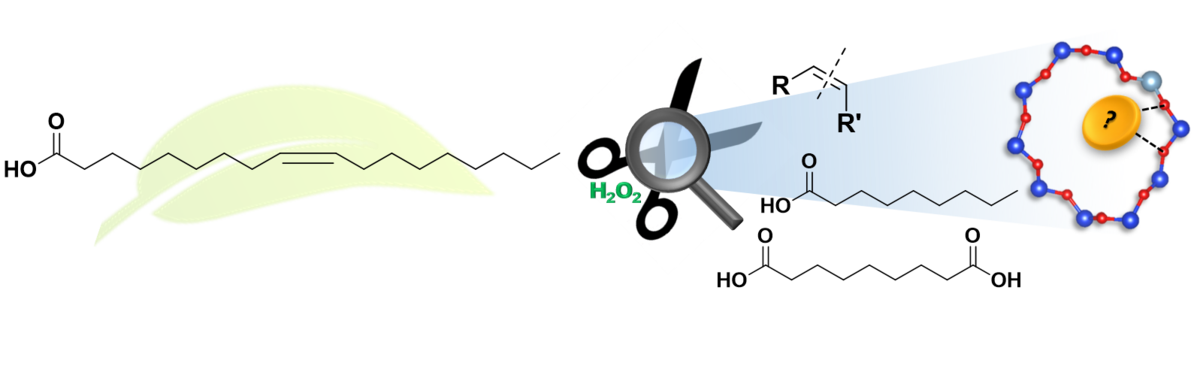
Catalytic Scissors: Oxidative Cleavage of C–C Bonds with Zeolite Catalysts
A circular chemicals economy depends on converting bio-derived molecules into functional building blocks while minimizing waste and harsh reagents. Oxidative C–C bond cleavage offers a powerful route from renewable alkenes (e.g., fatty-acid derivatives, terpenes, lignin-derived fragments) to aldehydes and carboxylic acids with broad industrial relevance. The central challenges are controlling selectivity versus over-oxidation, operating under mild conditions, and understanding which catalyst sites drive bond scission. We address this by designing well-defined metal–zeolite catalysts where redox functionality and acidity can be tuned independently, and by linking performance to catalyst structure through rigorous characterization and activity studies. This “catalytic scissors” strategy enables selective transformations that align with green-chemistry principles.
Related Publications
Enhancing the oxidative cleavage of vicinal diols on Fe-ZSM-5 catalysts with hierarchical porosity.
One-Pot Cascade Reaction from Epoxide to Carboxylic Acids Using Bifunctional Fe-ZSM-5
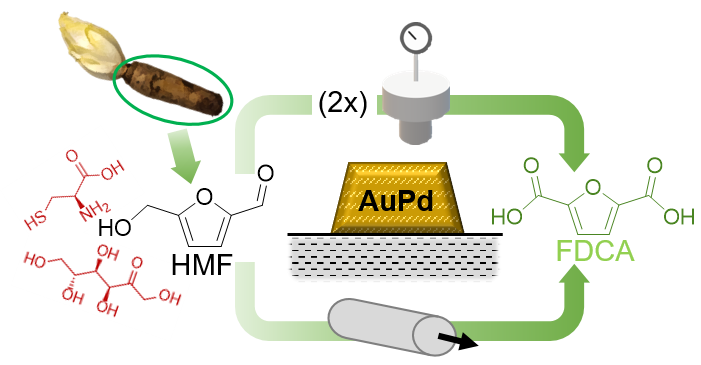
Knowledge-Based Catalyst Design for Valorization of Bio-Based HMF to FDCA
Sustainable plastics and packaging require renewable monomers that can compete in performance and scalability with petrochemical counterparts. FDCA, produced from biomass-derived HMF, is a key target, but practical implementation is limited by the complexity of real feedstocks and the sensitivity of oxidation catalysts to impurities and by-products. Maintaining activity and selectivity while reducing precious-metal dependence and minimizing purification steps remains a major open challenge. Our work takes a knowledge-based approach: we systematically map how realistic bio-based streams influence catalyst stability and reaction pathways, and we translate these insights into improved catalyst formulations and process conditions. This enables more resilient HMF-to-FDCA conversion concepts that bring bio-based monomers closer to industrial viability.
Related Publications
Selective Oxidation of Lower Olefins
↵
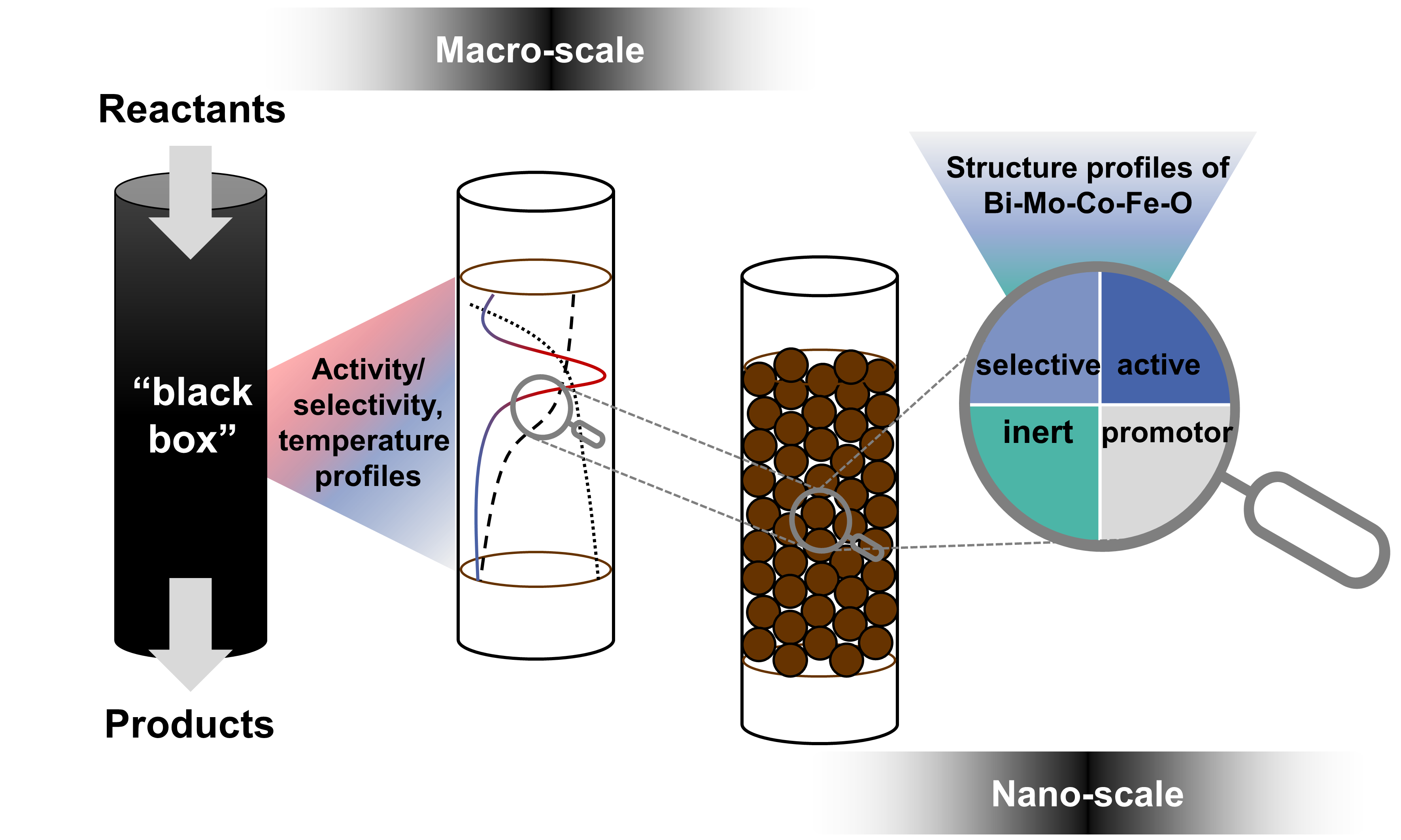
Efficient use of carbon resources—whether fossil-derived or increasingly bio-/waste-derived—relies on selective catalytic oxidation to generate high-value intermediates with minimal by-products. The oxidation of propylene to acrolein and isobutene to methacrolein underpins major chemical value chains, yet achieving high selectivity at industrially relevant conversion is difficult due to complex reaction networks, heat effects, and the dynamic nature of multicomponent oxide catalysts. We tackle these challenges by establishing structure–activity–selectivity relationships in bismuth molybdate-based systems, extending from well-controlled base compositions to more complex multimetal oxides. By integrating catalyst synthesis, kinetic testing, and advanced (including operando) spectroscopic and diffraction methods, we uncover governing principles that guide rational catalyst improvement—supporting more efficient and sustainable oxidation processes.
Related publications (selection)
L. Klag, T. L. Sheppard and J.-D. Grunwaldt, ChemCatChem 2023, 15, e202201276.
P. Sprenger, W. Kleist, J.-D. Grunwaldt, ACS Catal. 2017, 7, 5628-5642.
Available positions for students
| Topic | Title | Type | Supervisor |
|---|---|---|---|
|
Base-Free Glucose Oxidation on Zeolite-Supported Gold Catalysts |
tbd | Bachelor/ Master Thesis | Dr. Erisa Saraci |
| Knowledge-based Catalyst design for Valorization of Bio-based HMF | tbd | Bachelor/ Master Thesis | Maya Ludwig |